قلم یکبار مصرف الکتروسرجری دنا، محصولی است که برای برش آسان موضع جراحی، انعقاد سریع خون در اعمال جراحی و حذف بافتها و یا ضایعات پوستی استفاده میشود. استریل و یکبار مصرف بوده و با تمامی دستگاههای الکتروکوتر موجود در بازار سازگار است.














قلم یکبار مصرف الکتروسرجری دنا، محصولی است که برای برش آسان موضع جراحی، انعقاد سریع خون در اعمال جراحی و حذف بافتها و یا ضایعات پوستی استفاده میشود. استریل و یکبار مصرف بوده و با تمامی دستگاههای الکتروکوتر موجود در بازار سازگار است.
قلم یکبار مصرف الکتروکوتر یکی از پرمصرف ترین و کاربردیترین تجهیزات اتاق عمل میباشد.
از قلم یکبار مصرف الکتروکوتر برای ایجاد برش در بافت بدن یا جلوگیری از خونریزی بیمار در اتاق عمل مراکز درمانی و بیمارستانی استفاده میشود.
این وسیله به علت کاربرد مهمی که دارد، از مواد با کیفیت بالا و مدیکال گرید ساخته شده و استریل میباشد.
Conclusion: This Product is biocompatible.
D.G.Dena guarantees that this product is in conformity with Directive 93/42/EEC and that it has been manufactured following the directives of the Quality Assurance System certified as ISO 13485.
This product is classified as:

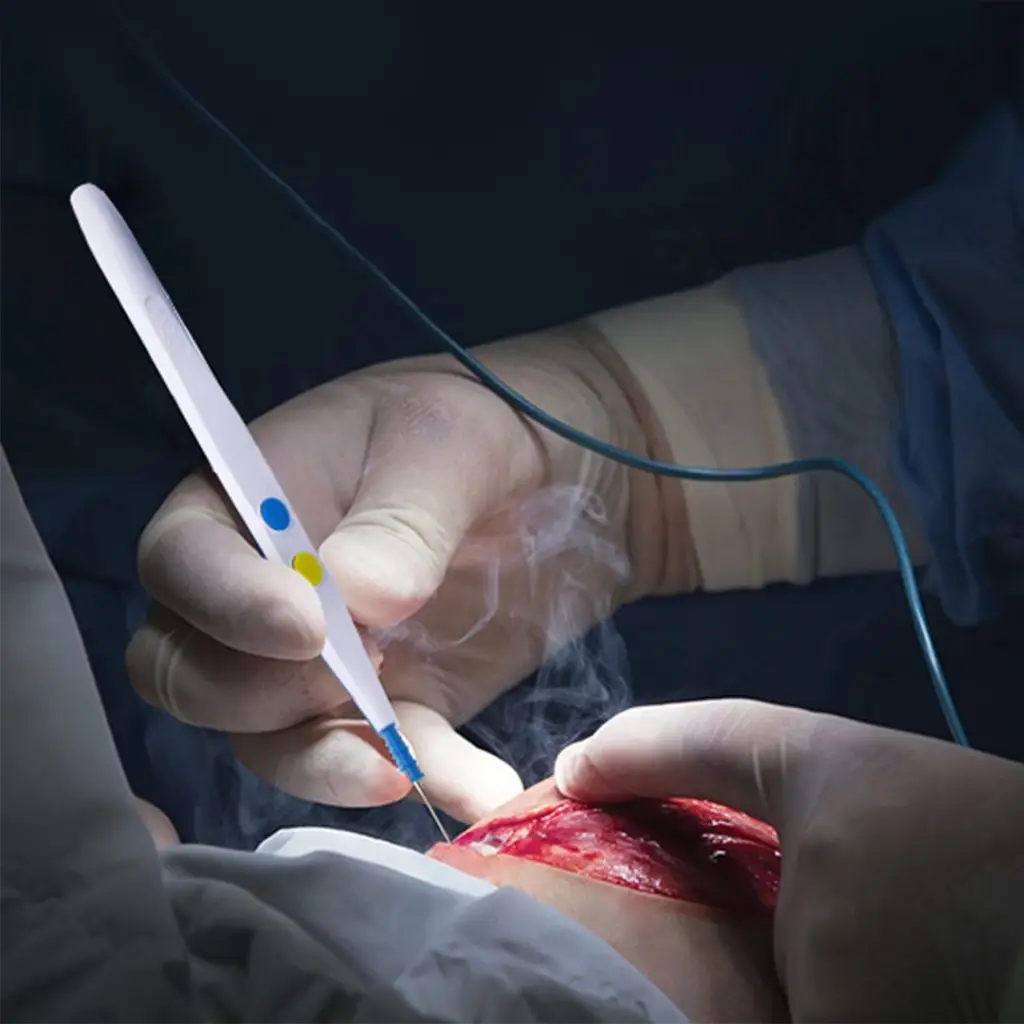
قلم یکبار مصرف الکتروکوتر دنا، یک وسیله پزشکی برای برش بافت بدن بیمار، حین جراحی است. این ابزار جراحی حین برش، عروق خونی را بسته و به همین دلیل توانسته تا حدود زیادی تهاجم را در عملهای جراحی کاهش دهد. این وسیله پزشکی کاملا یک بار مصرف میباشد؛ دلیل این امر آن است که از شیوع و انتقال میکروب و عفونت جلوگیری کند.
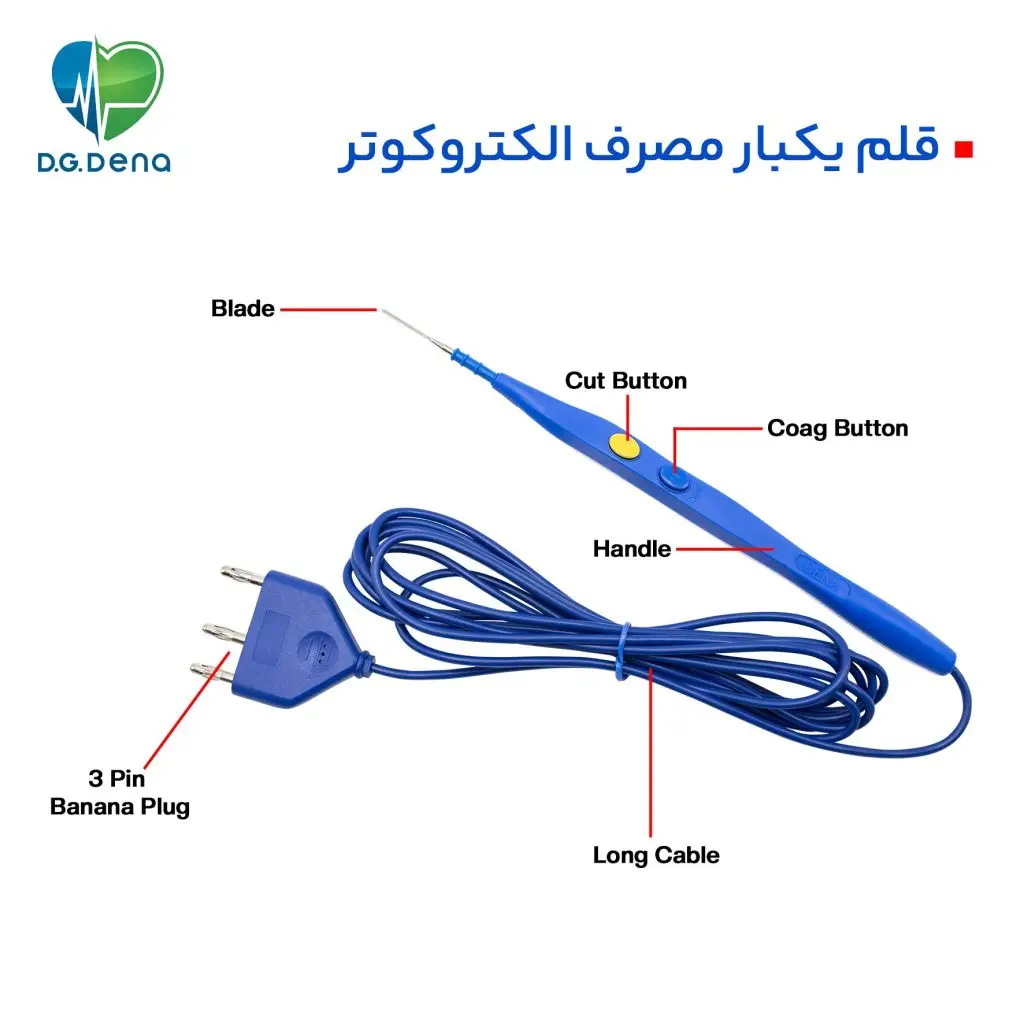
قلم یکبار مصرف الکتروکوتر دنا، با استفاده از جریان الکتریکی قابل تنظیم توسط دستگاه الکتروسرجری، شروع به برش بافت و انعقاد رگها و عروق خونی میکند.
بر روی این وسیله جراحی، 2 دکمه زرد و آبی به نامهای CUT (برش) و COAG (انعقاد) تعبیه شده است.
این قلم الکتروسرجری کاملا استریل (استریل شده با اتیلن اکساید) و ایمن بوده و جراح به راحتی میتواند حین عمل جراحی از آن استفاده کند.
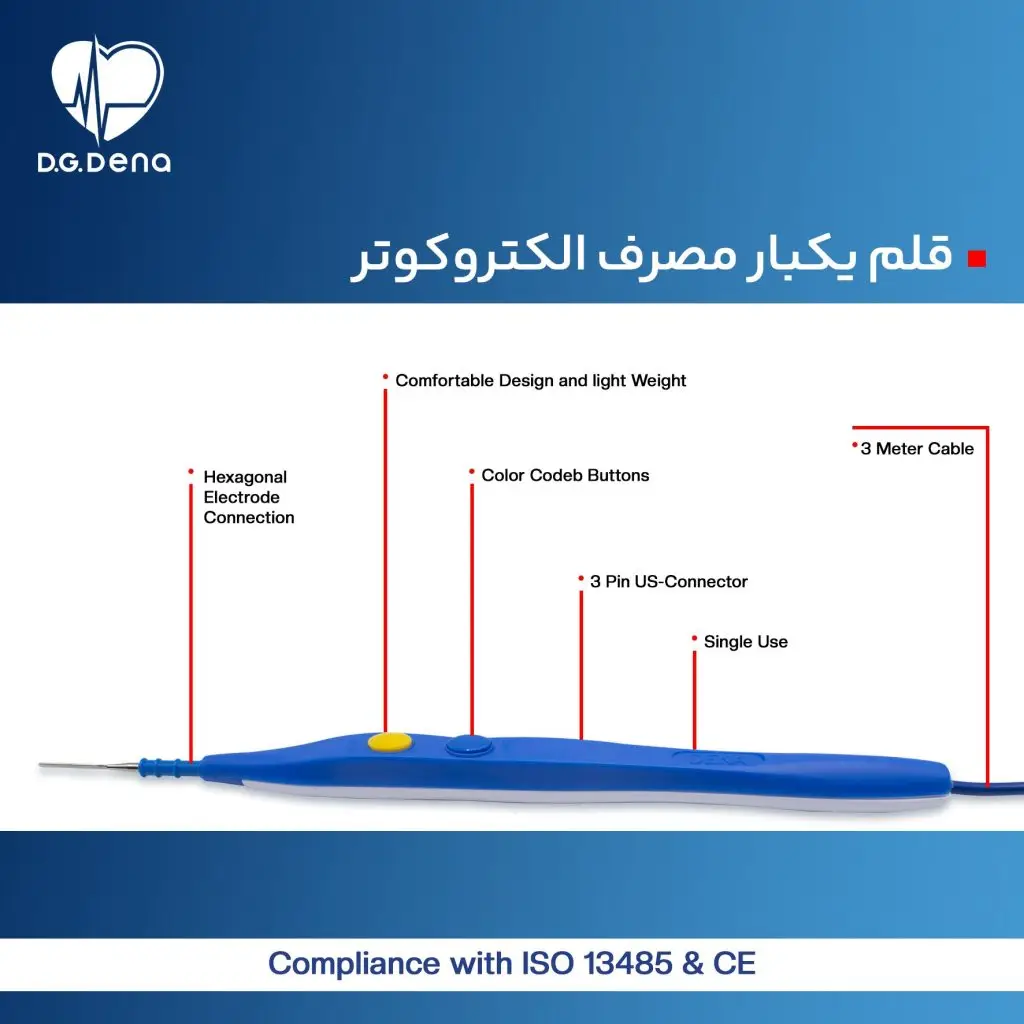
قلم یکبار مصرف الکتروکوتر دنا، به صورت کاملا یک بار مصرف و استریل طراحی شده و طراحی سبک و غیر لغزشی آن، باعث استفاده راحت و آسان توسط پزشک جراح خواهد شد.
به صورت کلی، ویژگیهای قلم الکتروسرجری دنا به شرح زیر است:
امروزه قلم یکبار مصرف الکتروکوتر دنا، باعث راحتی عملکردهای برش در پروسه جراحی شدهاند و به پزشک جراح کمک میکنند تا عمل جراحی را با سرعت و دقت هرچه تمامتر انجام دهد. به طور کلی میتوان گفت که از قلم یک بار مصرف جراحی برای موارد زیر استفاده میکنند:
ابتدا باید نوع کاربرد قلم را مشخص کرده و سپس با توجه به آن اندازه تیغه، سازگاری با نوع دستگاه الکتروسرجری و تولید کننده، اقدام به خرید این وسیله کرد.
قلم الکتروسرجری یک بار مصرف دنا، دارای یک تیغه استیل بوده که توسط جریان الکتریسیته بافت را برش می زند و میتواند باعث انعقاد در عروق بافت مورد نظر گردد و خونریزی را متوقف کند.
کار کردن با قلم الکتروسرجری یک بار مصرف دنا، بسیار آسان بوده و به دلیل وزن کم و طراحی ارگونومیک، استفاده از آن برای جراح بسیار راحت می باشد. جراح به راحتی با اتصال قلم به دستگاه الکتروسرجری ، میتواند عملیات برش و انعقاد را انجام دهد.
قلم یکبار مصرف الکتروکوتر دنا، کاملا استریل و یک بار مصرف بوده و پس از آن دیگر قابلیت استریل شدن ندارد.
برای خرید قلم یکبار مصرف الکتروکوتر دنا، از طریق شمارههای ثبت شده در وبسایت، با ما تماس بگیرید.