The DG DERM™ Barrier film is a transparent, breathable, fast-drying skin barrier spray that forms a durable protective layer on healthy or damaged skin. It protects skin from exposure to urine and feces, medical adhesives, trauma and friction in bedridden patients. Application is non-stinging and well-tolerated.
Applications of DG Derm™ Barrier Film:
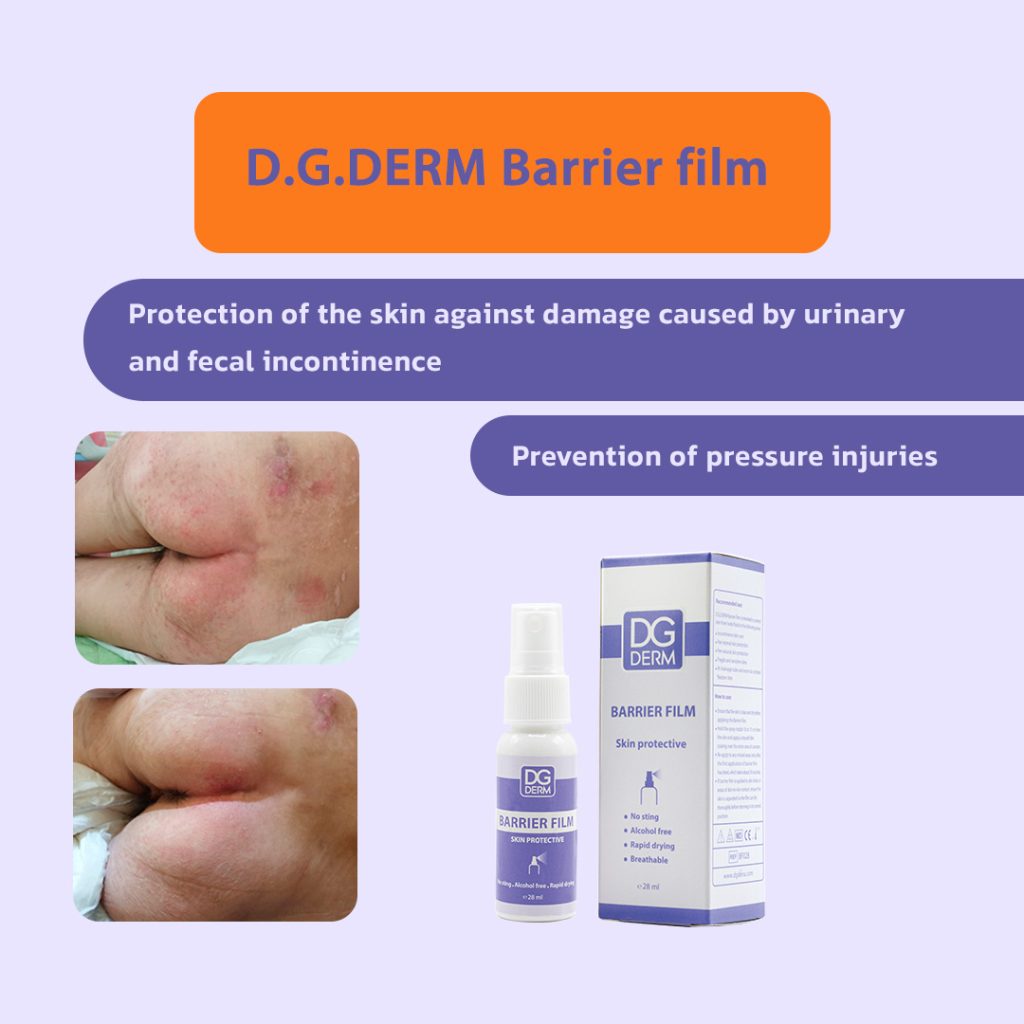
IAD (Incontinence-Associated Dermatitis):
In incontinent patients, direct contact of urine or feces with skin causes abnormal softening (maceration), redness, itching and pain. DG Derm™ Barrier Film acts as a semi-permeable barrier that repels excess moisture and prevents enzymes and salts present in urine/feces from contacting the skin directly, thereby reducing skin damage.
MARSI (Medical Adhesive-Related Skin Injury):
Tape and adhesives can stretch and abrade the epidermis during dressing change or adhesive removal, leading to MARSI. DGDerm™ Barrier Film creates a protective layer that prevents direct contact between adhesives and epidermis, minimizing epidermal stripping on removal.
Protection at CVC and PICC sites:
Central venous catheters (CVC) and peripherally inserted central catheters (PICC) are fixed to the skin with medical adhesives. Tension and pressure from adhesives may microbleeds or small blisters.
DG Derm™ Barrier Film allows the adhesives to adhere the silicone-like film instead of the epidermis, which can reduce friction and mechanical stress. This lowers the risk of contact dermatitis and secondary infection.
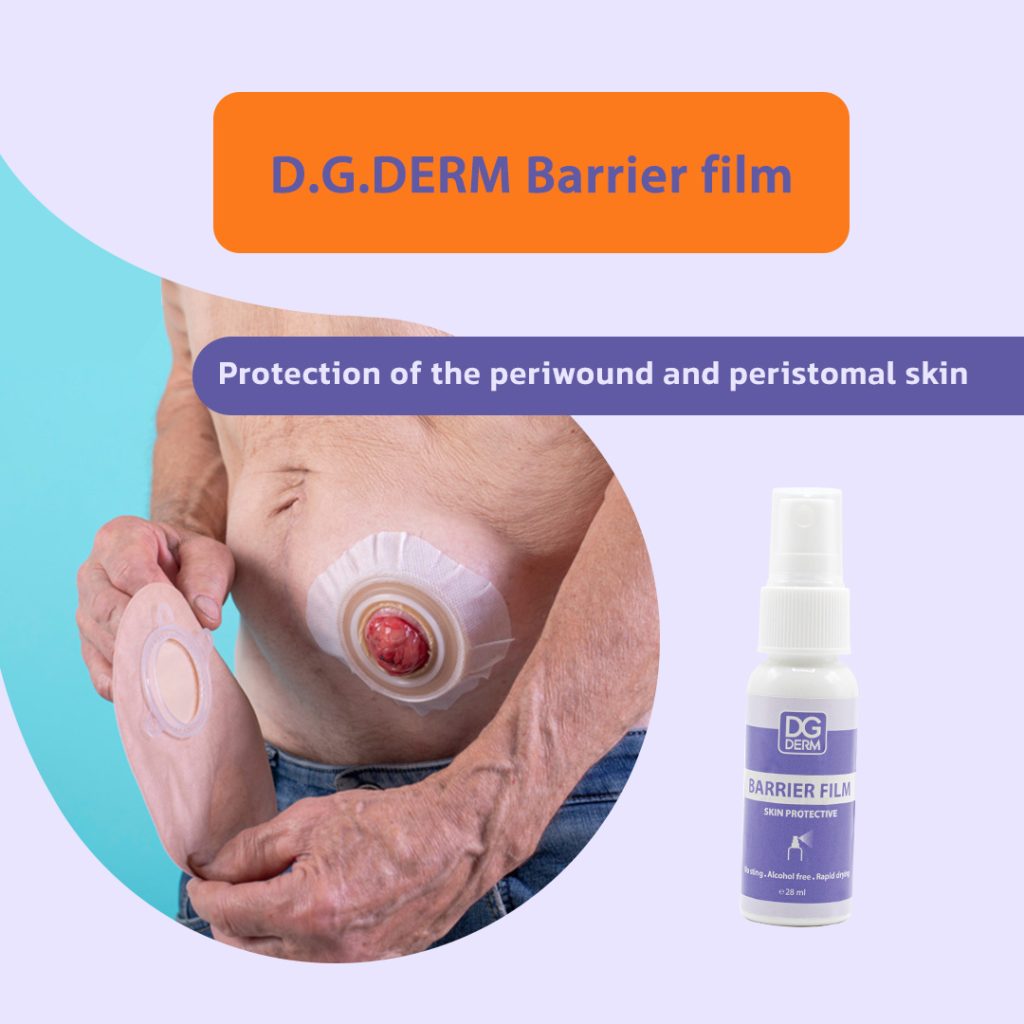
Stoma care (peristomal protection):
Patients with stomas (colostomy, ileostomy, urostomy) may experience redness or irritation from prolonged contact with adhesive products.
DG Derm™ Barrier Film forms a transparent protective layer around the stoma to prevent damage caused by ostomy adhesives.
Radiation therapy skin protection:
Ionizing radiation used in radiotherapy can cause dry, peeling and reddened skin in the irradiated area. Maintaining relative moisture and creating a protective layer can reduce the severity of radiation-induced skin reactions and relieve patient discomfort.
Applying DG Derm™ Barrier Film during the course of radiotherapy and for several days after sessions helps protect the irradiated skin by preserving moisture, reducing surface abrasion, and minimizing pain and radiodermatitis.
Suitable for sensitive skin and vulnerable groups:
- Neonates and infants: Their skin is very thin and delicate. DG Derm™ Barrier Film is formulated without alcohol and latex, making it suitable for protecting areas prone to irritation (e.g., monitoring lead wire sites or vascular access sites) in neonates and children.
- Older adults: Thinning of the epidermis and reduction of skin elasticity in elderly patients, increases the risk of microscopic tears and bruising during dressing changes. DG Derm™ Barrier Film helps distribute pressure and reduce mechanical stress.
How to use DG Derm™ Protective Film: DG DERM™ Barrier film
- Ensure the skin is clean, dry, and free of oils or lotions.
- Hold the spray 10–15 cm (4–6 inches) from the skin and apply evenly.
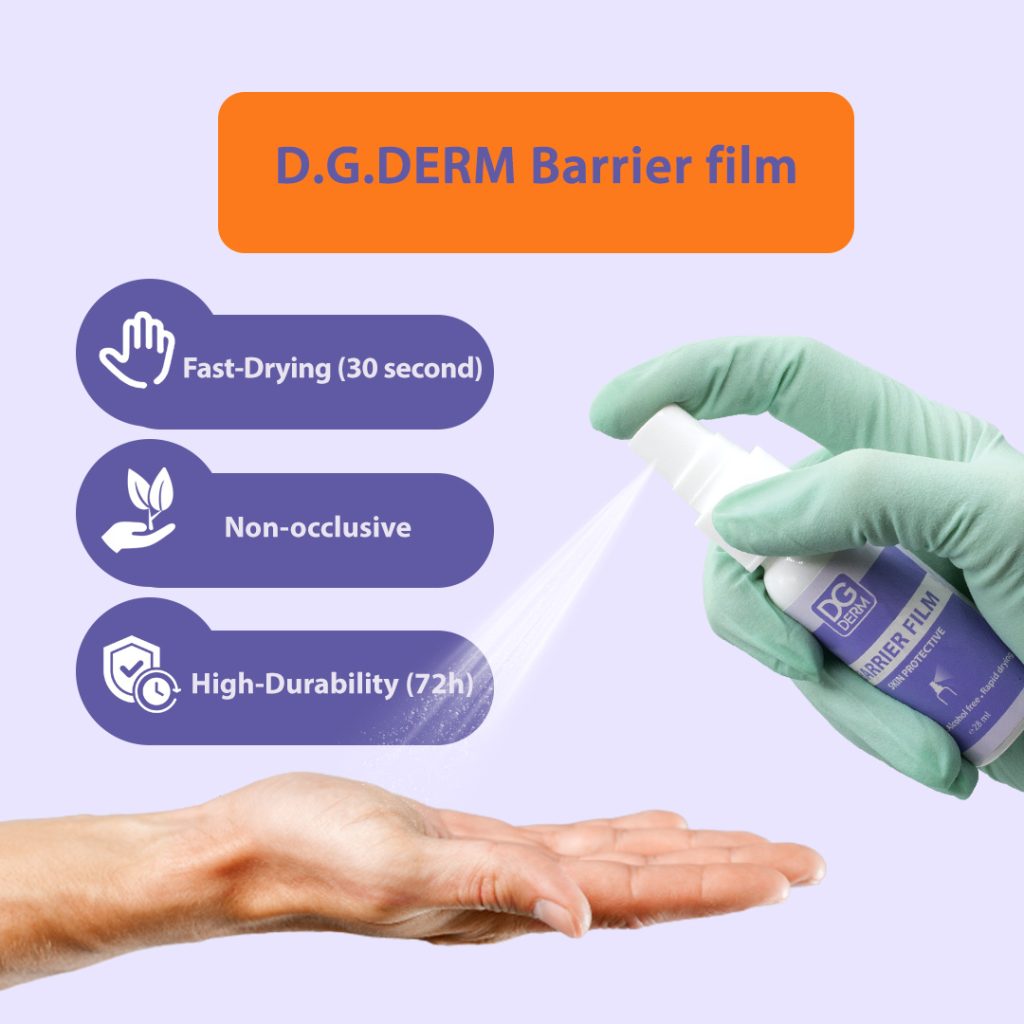
- The film dries within approximately 30 seconds and forms a stable, transparent barrier on the skin.
Re-application intervals:
- Under dressings or medical adhesives: Reapply at each dressing change (maximum every 72 hours). The film remains effective against washing and friction for up to 72 hours.
- Incontinence prevention: Clinical protocols indicate that three applications per week (every other day) are effective and cost-efficient.
- Radiation therapy: Apply 2–3 times per week according to the local radiotherapy center protocol.
Compatibility with antiseptics: After drying, the DG Derm™ film is compatible with antiseptic solutions, including chlorhexidine gluconate (CHG), and does not interfere with their activity.
Warnings and safety:
- For external use only. Avoid contact with eyes, mucous membranes, and open wounds.
- The product is alcohol-free, fragrance-free, and preservative-free; therefore, the risk of irritation and contact dermatitis is very low. It has been reported safe for use on highly sensitive skin and acne-prone skin.
- Discontinue use and consult a physician if unusual irritation or allergic reaction occurs.
- Flammable: Keep away from heat sources and open flame until the product is fully dry.
Clinical evidence and documented results:
- Reduction of adhesive-related erythema (redness) by up to 96%.
- Prevention of maceration in 94% of cases.
- Reduction of pain associated with epidermal stretching during adhesive removal.
- In clinical studies of patients with MARSI, MASD and catheter-related skin injury, use of DG Derm™ Barrier Film during treatment, increased the average duration of intact skin from 11.7 days to 14.5 days (approximately a 25% increase).
- In the same clinical data set, moisture-related skin injuries were reduced from 16 cases to 2 cases, adhesive residue-related redness decreased from 6 cases to 1 case, and skin breakdown on removal decreased from 19 cases to 2 cases — representing an approximate 80% reduction in potential skin injuries.
Stability and durability:
The DG Derm™ Barrier Film protective film provides durable protection against moisture and friction; typical in-use durability is up to 72 hours under normal conditions. (See technical documentation for detailed stability graphs.)
How to purchase:
To purchase DG Derm™ protective film, please contact the phone numbers listed on our website or contact your local distributor.
Barrier Film highlights:
- Extremely fast drying (about 30 seconds)
- Non-stinging, well tolerated
- Transparent protective layer
- Breathable yet water-resistant
- Economical and long-lasting protection (up to 72 hours)
- Transparent for easy visual monitoring
- Does not reduce effectiveness of ostomy adhesives
- Improves adhesion of dressings and tapes
- Resistant to washing, reducing the need for frequent re-application
- Alcohol-free and free of common sensitizers