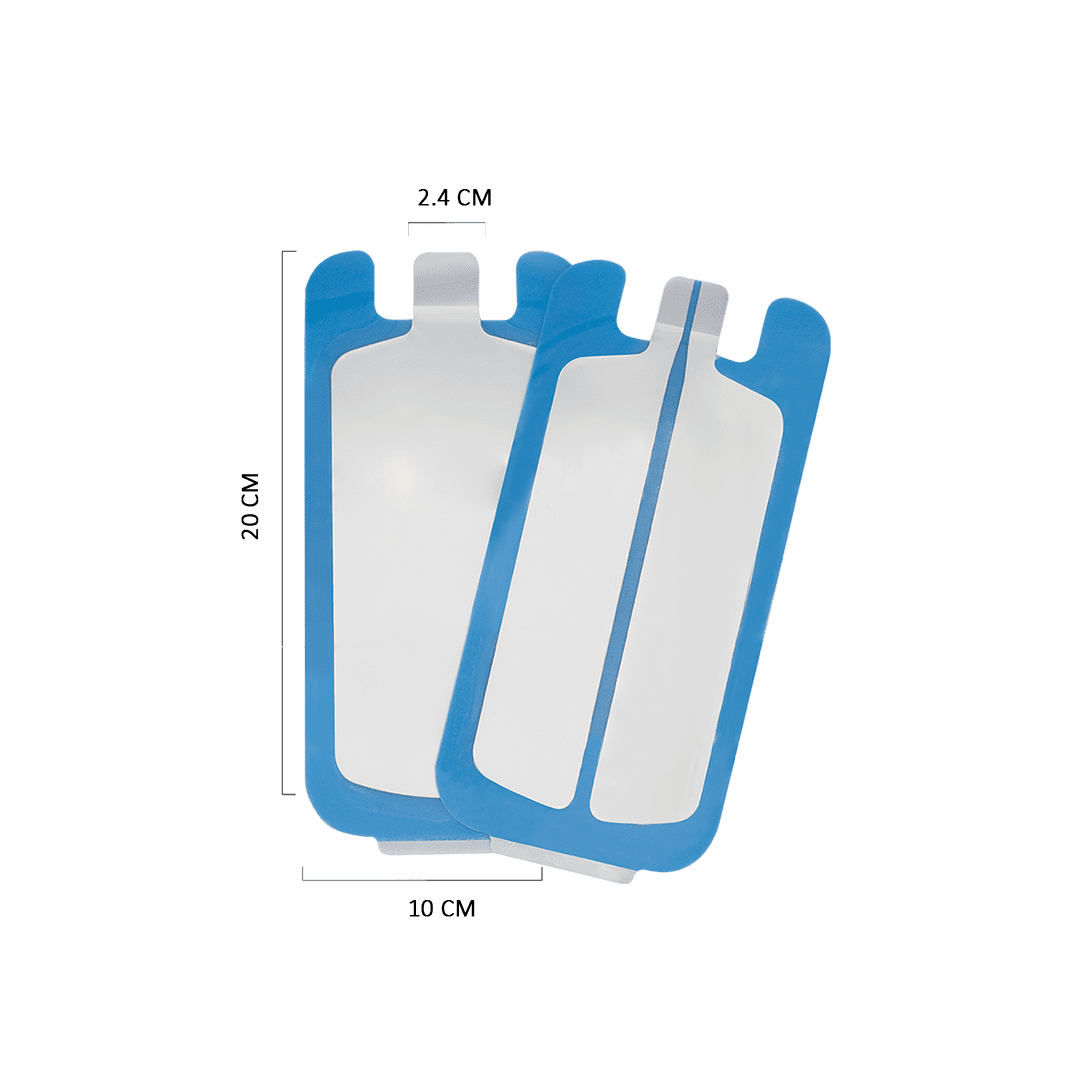
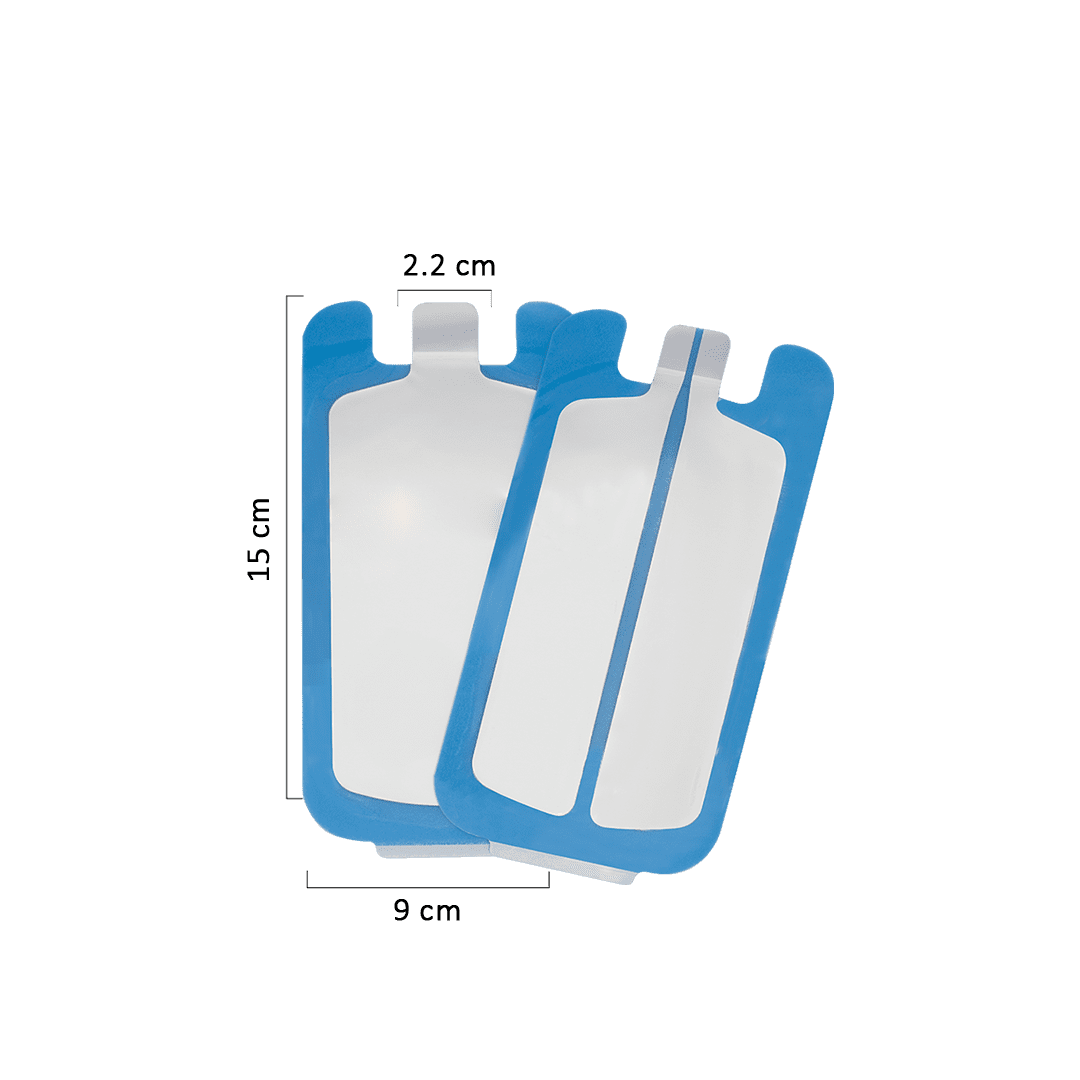
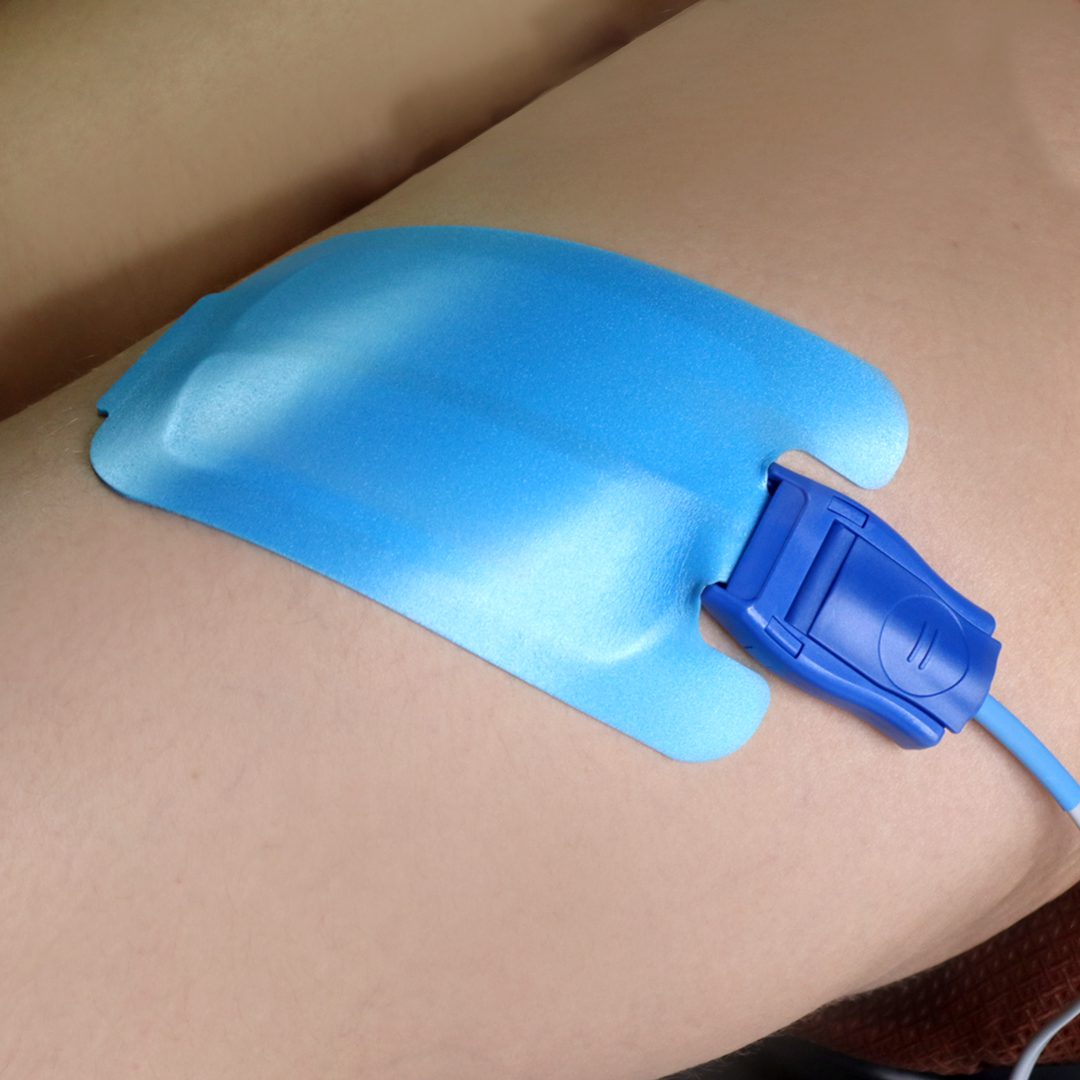
An Electrosurgical Grounding Pad is a soft and flexible pad that completes the electrical current circuit in electro-surgical procedures. The flexibility of this plate allows for comfortable use on all body areas.
This Grounding Pad delivers the electrical current back to the electrocautery device through an electrode and its connected wire after the incision and cauterizing. This prevents the patient from getting burned.
This product is made with high-quality materials and available in two models of Split & Non-split, suitable for all electrocautery devices. It has obtained certification for all relevant safety and biocompatibility tests.
A continuous metal plate collects and conducts the input current from its surface to an external circuit in the non-split model. On the other hand, the split configuration offers enhanced safety by directing the accumulated electrical current through both plate edges. The other edge compensates by ensuring the continued conduction of the electrical charge, if one edge fails.
Direction for Use
- The patient’s body should be clean, with no hair and oil.
- Choose the proper plate size.
- Remove the transparent layer and stick it onto the patient’s body.
- The patient should remove all accessories and metallic objects.